Minimal risk studies pose little to no risk to study participants. Your research most likely qualifies as minimal risk if you are collecting data from adults (18 years of age and older) through surveys, questionnaires, focus groups, interviews, non-invasive or non-violent games, passive observation of public behavior, etc. IRB will likely consider your research to fall into their "exempt" category.
This category requires the submission of forms:
- HRP 254 (consent)
- HRP 255
- and a copy of your instrument(s), in addition to an IRB application in HURON.
Regarding a copy of your instruments: A copy of your instrument(s) could be a word document with your survey, interview, questionnaire, or focus group questions. If you are using a licensed product, such as a test, evaluation, or assessment tool, include a document with the test questions. If you are using a device, such as a heart rate monitor or some other exercise device, include a description of it, including how it will collect data from subjects. IRB may require you to upload a picture and/or a professional description of the device.
Submit form HRP 255 in the protocol section of the application:
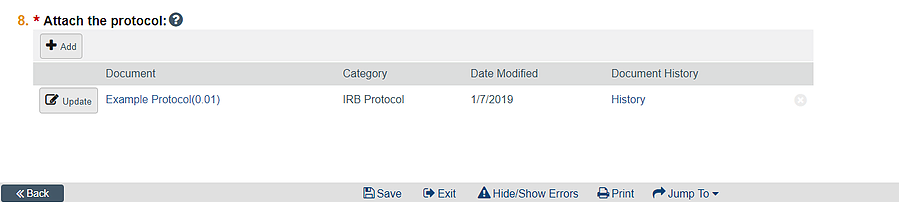
Submit form HRP 254 in the consent section of the application:
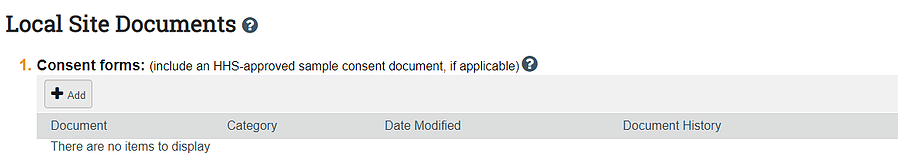
Submit the copy of your instrument(s) in the other documents section of the application:
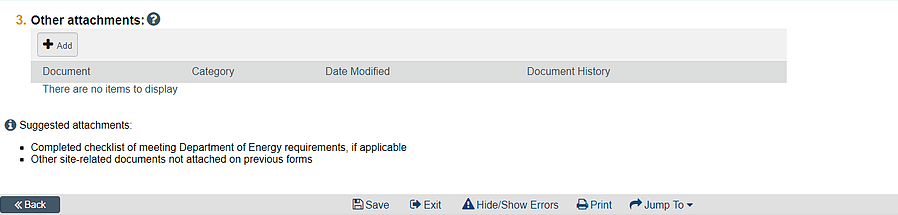
Please plan ahead for any issues that may cause a delay in IRB approval.
Still stuck? Contact us:
CCIERAST@ucf.edu
407-823-4280